How to Do a Bomb Calorimeter Problem
Brown fat has a protein that uses the food we consume to do what instead of creating energy in the form of ATP. A 691 mol sample of ethylene is burned in a bomb calorimeter that has heat capacity of 3480 JC-1.

Bomb Calorimetry Problem Chemistry Youtube
To do this you will use a homemade bomb calorimeter that captures and measures the heat the energy flow associated with differences in temperature released by burning food.

. The temperature of the water jacket rises from 2483C to 3192C. Calculate Delta E for the combustion of one mole of ethylene. In this article you will find plenty of interesting math topics.
Mass of benzoic acid tablet. 1- basal metabolism 2- physical activity. You can even use mathematical writing as a tool in problem-solving.
To generate heat for. In the center of the water bath is the bomb cell which. Mathematics is the science of numbers and shapes.
A student runs two experiments with a constant-volume bomb calorimeter containing 1300. The three main purposes that the body uses energy for. The basic idea of a calorimeter is to release all the stored energy at once and capture all the released heat with a reservoir of water.
A bomb calorimeter has a water bath with a stirrer and thermometer inserted. Besides you will learn about. Writing about it can give you a fresh perspective and help to clarify difficult concepts.

Chemistry Thermochemistry 27 Of 37 Combustion In A Bomb Calorimeter Ex 1 Youtube

Bomb Calorimetry Hw Example Youtube
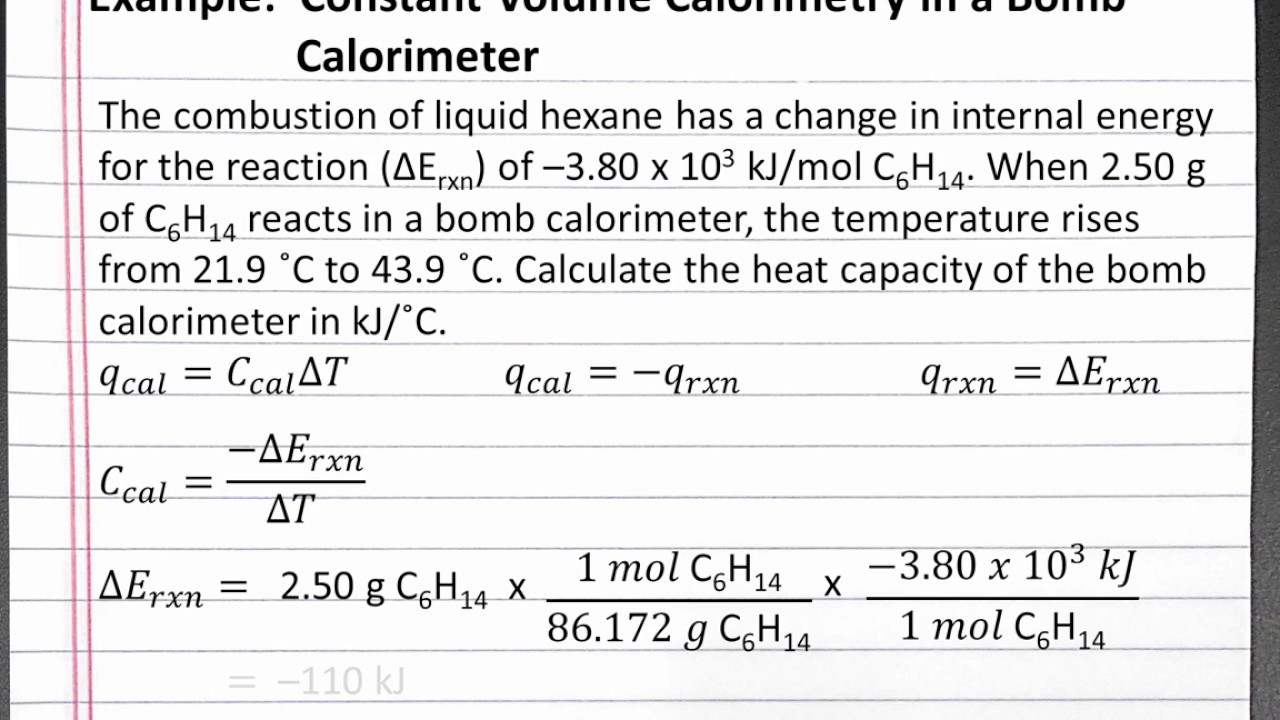
Chemistry 101 Calculating Heat Capacity Of A Bomb Calorimeter Youtube
No comments for "How to Do a Bomb Calorimeter Problem"
Post a Comment